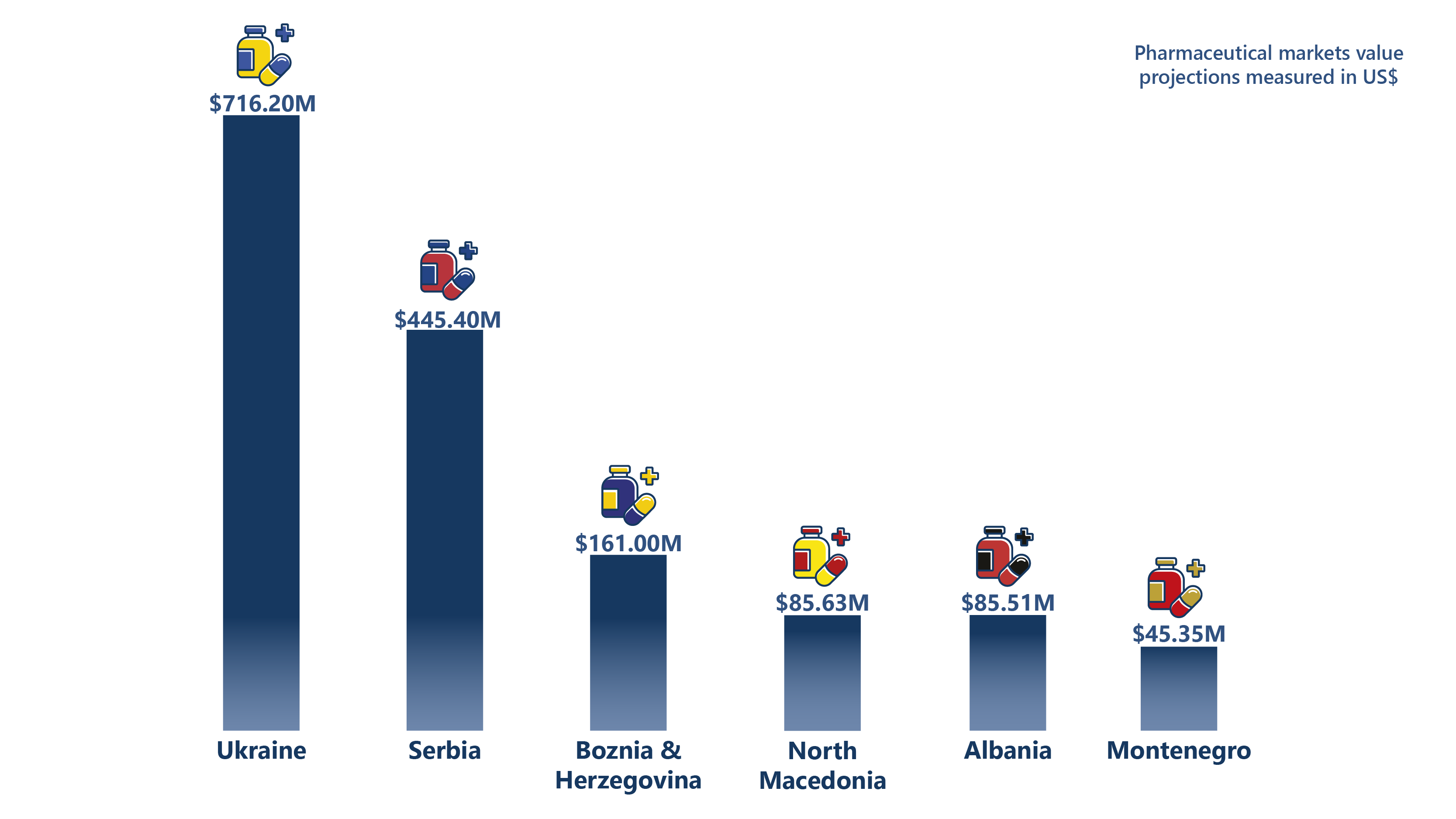
The pharmaceutical sectors in Ukraine and the Western Balkans are critical to the region's healthcare landscape and economic development, though they vary significantly in size and capacity. In 2024, the pharmaceutical market is projected to generate US $716.20 million in Ukraine, US $445.40 million in Serbia, and US $161.00 million in Bosnia and Herzegovina, reflecting substantial potential for growth and development. Smaller markets, like Montenegro, Albania, and North Macedonia, also show promising trends, with revenues expected to reach US$45.35 million, US $85.51 million, and US $85.63 million, respectively.

The project Mapping Pharmaceutical Manufacturing Facilities in Ukraine and the Western Balkans, funded by the European Commission, aims to support the EU's strategic autonomy and address critical medicine shortages. This initiative focuses on creating a detailed inventory of pharmaceutical manufacturing capabilities in Ukraine and six Western Balkan countries (Albania, Bosnia and Herzegovina, Kosovo, Montenegro, North Macedonia, and Serbia). The study identifies manufacturers of therapeutics, vaccines, diagnostics, and personal protective equipment (PPE), as well as upstream facilities producing active pharmaceutical ingredients (APIs), to enhance the EU's capacity for supply chain diversification and resilience. A mapping is conducted to build a robust inventory of the capabilities of pharmaceutical manufacturing facilities in Ukraine and the Western Balkans. In alignment with EU cross-cutting themes, like environmental sustainability and a human-rights-based approach, the project emphasises equitable benefits for local and EU populations. It seeks to uncover vulnerabilities in the supply chains of critical medicines and develop recommendations for mitigating risks through manufacturing diversification and capacity building.
Implementation
To support the development of a study to evaluate pharmaceutical supply chain resilience, identify diversification opportunities, propose solutions for regulatory alignment, and enhance local production capacities, NTU team applies a comprehensive multi-phased approach. More concretely, NTU team contributes to:
-
Conducting a desk review to analyse regulatory environments, supply chain dynamics, and EU import standards, supported by key databases (e.g., EMA, WHO, FDA);
-
Establishing strategic contacts with Ministries of Health and other government bodies to conduct interviews, gather insights, and align objectives;
-
Organising stakeholder consultations with pharmaceutical associations, healthcare providers, international health organisations, NGOs, etc.;
-
Organising field visits to selected facilities to assess production capabilities, quality standards, adherence to EU Good Manufacturing Practice standards, and engage with key stakeholders;
-
Drafting a report synthesising all collected data, mapping key manufacturers, evaluating development possibilities, and providing regulatory recommendations;
-
Facilitating a consultation process to present the study's findings to a wider community of stakeholders and update the study based on their feedback.
Impact
Global impact of the project includes:
-
Enhancement of supply chain resilience by identifying vulnerabilities and proposing actionable solutions to strengthen the stability and reliability of pharmaceutical supplies.
-
Promotion of diversification of production sources to reduce dependency on a limited number of suppliers and create a robust and flexible pharmaceutical supply chain, capable of adapting to unexpected challenges.
-
Improved regulatory alignment by provision of a thorough analysis of existing frameworks and offering recommendations for enhancements.
-
Increase of local production capacities, helping boost economic growth, create jobs, and improve access to essential medicines.
-
Fostering regional cooperation, particularly in the Western Balkans, to help leverage shared resources and expertise, creating a more integrated and competitive pharmaceutical sector in the region.

SDGs:






